Dysport: The “New” and Better Botox
by Lawrence Janowski, MD
Botox® was the first neurotoxin approved for cosmetic use in 2002. Since then, two virtually identical products have been introduced: Dysport® and Xeomin. All three neurotoxins are Botulinum Toxin type A, and do exactly the same thing, last about the same amount of time, and, until now, cost the same. Dysport® was FDA approved in 2009 (so it’s not really new), and since then has been growing in use. I began offering it last year to my patients, and I’m very impressed with it.
A logical question would be: “Why offer another product that does exactly the same thing as Botox®?”. The main reason: Price. I am able to offer Dysport® at a 10% discount relative to Botox®, while achieving the exact same result. But as I began to treat patients with it, I also noticed some very interesting things. Patients are reporting that it kicks in quicker, perhaps at 2 days compared with Botox® at 4-5 days. Another interesting phenomenon is that some long-time Botox® users have been complaining to me that they feel their Botox® “just doesn’t work as well as it used to”. I find I am doing more touch-ups and using higher quantities to achieve the same effect. After switching these patients over to Dysport®, miraculously they are reporting they are getting the full effect that they used to get, and they are very pleased with it. Some patients are finding it lasts longer than Botox® as well.
After hearing so much positive feedback, I now believe Dysport® to be a superior product to Botox®, and I am recommending it to all of my clients. If I can achieve as-good or better results for less money, why wouldn’t I?
Liz Janowski
Related Posts

Less Bruising & Pain is Possible at Sonata
We truly know how nervous people can be before their first aesthetic treatment. And we have so many ways to help...

Lips are In
Full, beautiful naturally delicious-looking lips are now an option for almost anyone. Enhancing your lips is both an...
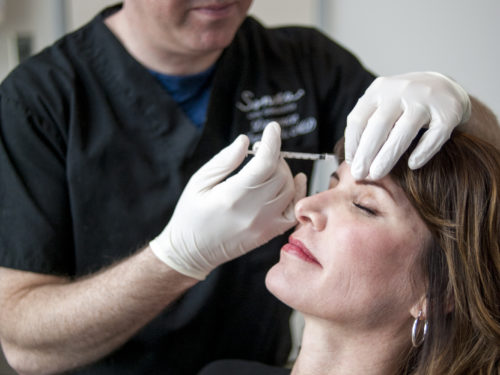
My First Time – Botox/Dysport
Every day, we have clients visit our Broomfield, Colorado office who are trying Botox®/Dysport® for the first time....

